TDII
Texture based diffuse illness investigation in ultrasound imaging
This work is funded by the Romanian National Authority for Scientific Research as part of the CEEX program FINALISM 94/31.07.2006 and SIDEF 71/17.07.2006
Introduction
Image Processing techniques are more and more used in medical diagnosis field. One particular area where these techniques are employed with success is the analysis of the ultrasound images. Ultrasound examination is very popular because it is non-invasive and relatively cheap. However it cannot be used in all situations. Diagnosing the diffuse liver diseases is one area where ultrasound examination meets its limits because the visual aspects of healthy/affected liver are very similar. Texture analysis is used to study ultrasound images and to help diagnosing diffuse liver diseases.Texture analysis implies computing some texture attributes and classify the textures using a classification algorithm. The basic approach is to compute these texture features from a rectangular region (Region of Interest, ROI) and use them to classify the image. There are some issues that prevents a straightforward approach.
At liver tissue there are four pathologies that coexists. In this research field liver biposy is used as ground thruth. Using biopsy these pathologies can be assessed. Unfortunately liver biopsy is an invasive procedure with various side effects (pain, bleeding, even death) [Saadeh_2000]. For this reason the number of available patients that can be included in the study is relatively low. Some authors point out that even the biopsy suffers from some sampling errors in a magnitude of 25% [Saadeh_2000],[Poynard_2000]
The diseases that affects the liver coexists in various grades and stages. The distribution among the population of these diseases is not uniform neither independent. As a result there are some combinations of steatosis/fibrosis that are well represented (and well represented in our patient lots) and some of them that are scarce. This unequal distribution rises another issue because one way to analyse a disease is by starting with patients sufering only from this pathology. Unfortunately "pure" patients are scarce. We have to deal with mixed patient lots and try to asses two or three pathologies in the same time. In literature fibrosis and steatoshis are mostly the only two pathologies investigated. It is assumed that these pathologies have the most important impact in altering the aspects of an ultrasound image. [Yeh_2003],[Lu_1999]. In owr work we try to assess the effects of fibrosis, steatosis, necro-inflammatory processes and hepatocyte ballonisation.
Physicians acquire images using an ultrasound probe. The position and orientation of the probe with respect to human body have a dramatic impact on the content of the image. Althrough the ultrasound acquisition protocol specifies some constrains related to the position and orientation of the probe, the content of the US image varies greatly. In adition to that US image aspect varies from patient to patient and even within the images acquired from the same patient. Beside that, some patologies alter the volume of the liver. As a result the position of ROI is different for each image and have to be established manually. This limits again the avalilable number of samples because the medical expertise is used to establish the ROI for each image. Because the fact that a human person is involved, the process of establishing ROI's is a time consuming and subject to errors.
Another direction of the current research is establishing an automatic ROI selection methodology. Image segmentation is employed in order to identify the liver tissue and to determine a region of interest free of artifacts. Ultrasound images acquired from the liver have low contrast between liver tissue and the surrounding organs. For this reason image segmentation is very difficult. There are few papers that address this issue [Noble_2006].
Objectives
• Develop software tools fitted for the research area.
• Create a software system that allows a structured reporting on US images.
• The software system must have a friendly GUI because it will be used by physicians in ROI establishment.
• Implement and validate texture attribute computation algorithms found in literature.
• Develop algorithms based on the domain knowledge (i.e. Evaluation of the behaviour of ultrasound waves into the tissue, evaluation of the liver capsule shape, the liniarity of the blood vessels, etc)
• Implement/integrate a number of classification algorithms.
• On the aquired US images establish regions of interest.
• Study the impact of diffuse liver pathologies on texture features
• Build data sets fitted for this study.
• Evaluate if the studied pathologies alter the values of the texture attributes
• Evaluate which disease has the greatest impact in altering the US images.
• Evaluate in which way each pathology affects the attributes
• Evaluate if one can develop a way to discriminate between existing pathologies.
• Study the impact of fibrosis on texture features
• Build data sets fitted for this study
• Use the implemented features to evaluate the impact of fibrosis
• Evaluate the classification performance and inference if current methodology is fitted for fibrosis detection.
• Based on previous evaluation modify the current methodology and evaluate it.
• Build imagistic models for this disease that will accurately detect the fibrosis stage independent of other superimposing diseases
• Study the impact of steatosis on texture features
• Build data sets fitted for this study
• Use the implemented features to evaluate the impact of fibrosis
• Evaluate the classification performance and inference if current methodology is fitted for steatosis detection.
• Based on previous evaluation modify the current methodology and evaluate it.
• Build imagistic models for this disease that will accurately detect the steatosis grade independent of other superimposing diseases
• Create a software system that detects the steatosis grade and fibrosis stage independently of associated pathologies.
• Build imagistic models for each disease
• Train and evaluate classificators based on these models
• Provide an easy to use software tool for diffuse liver disease identification and stadialisation
• Investigate means of ultrasound image segmentation and its applications on automatic ROI selection.
Methodology
Image processing techniques are used to study ultrasound images and to improve the diagnosis value of ultrasound in diffuse liver diseases. Features are computed over images and the images are classified based on the computed feature vectors. We use texture features along with some non-texture characterisation of the images.
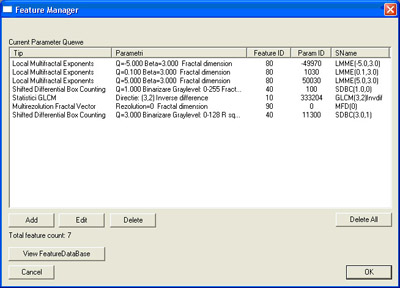
List of texture features implemented:
• Histogram statistics
• Gray tone difference matrix
• Gray level co-occurrence matrix statistics
• Multifractal differential box counting
• Local multifractal morphological exponents
• Multi resolution fractal dimension
• Law's texture energy measurements
• Wavelet transform
Non textural features study the behaviour of the ultrasound waves into the human body. They consists in attenuation and backscattering coefficients.
System architecture
The system consists of several modules and each one is responsible with different tasks. In figure 2 is shown the principal modules of the system and their imteractions.Local database module
Database module has a central role in storing information. Medical data and ultrasound images are handled here along with computed features. This module is the only one that interacts with other systems, mainly with the medical system.
One will focus on one disease at a time, or only at several aspects of the disease in order to better understand their effects on ultrasound image. Local database stores only the neccesary informations needed in these studies. For each such research step the database will be populated with required information. The information about the patients, their whole ultrasound image sets, demographic data, etc. are stored into the medical data system.
Feature extraction moduleThis module is responsible with feature computation. Here we implement the algorithms that extract features. The textures are selected from the ultrasound images by setting a rectangular region of interest on the image. Other algorithms require diferent kind of region of interests (i.e. line segment). Feature extraction module has to be able to process ultrasound images provided by the local database module along with ordinary gray scale images. There are reasons (like the validation of algorithms) that require to process ordinary images. Computed feature sets will be stored in the local database. For ordinary images, computed feature sets can be stored in flat files (CSV format).
Classification moduleThis module implements the classification algorithms. These algorithms are used in texture recognition and classification using extracted texture feature sets. Based on texture classification we classify the ultrasound images according to their pathology. There are a number of classification algorithms presented in the literature. One goal of this study is to identify which is the best fitted classification algorithm. In order to do so, one has to be able to asses the algorithm efficiency in terms of classification performance. Some of the algorithms are readily available on various open source image processing libraries. Each one has its different interface and different input/output data format. This module is also resposible with presenting the data in proprer format.



Achievements
|
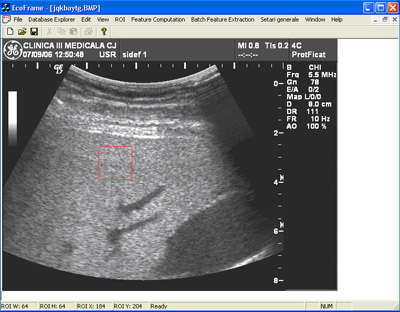
In figure 3, 4 and 5 are presented some aspects of the user interface. One should note the ease of use even for a person that is not familiarised with the project. Some parts of the software module are designed to be used by doctors. (i.e. patient information form, US image administration form, etc) Using this system we were able to succesfuly distinguish between healthy and severe steatosis patients with an accuracy of almost 100%.
Table 1. Discrimination rates between healthy patients and steatosis. The features used are attenuation and backscattering coeficients computed along a stragiht line on a US image. (see figure 6) These features globally evaluates the behaviour of ultrasound waves into the liver. The images were acquired from the right lobe at 16 cm depth, using the same settings of the ultrasound scanner.
In literature, texture features are used in order to assess the fibrosis stage. We employ nine algorithms that generate over 1200 features. Regarding fibrosis detection the results are less spectacular. Steatosis can mask out subjacent fibrosis and other pathologies like inflamation or necrosis (quantified as activity grade) affect the image. Good discrimination rates will be achieved by building imagistic models. These imagistic models will describe the image aspects for each combination of fibrosis stage, steatosis grade and activity grade. Unfortunately the distribution among patients of these pathologies is not uniform. Althrough we examined over 600 patients, there are pathology combination groups that do not contain enough patients to allow us a statistical significant study. |
 Figure 3. Database interface. Ultrasound image administration form
|
Conclusions and further developments
The software system presented here provides a number of very useful tools in detecting diffuse liver diseases.
Steatosis is best detected using features that evaluate the behaviour of US waves into the liver tissue. On texture features steatosis has also a great impact. When medium/severe steatosis is present it can mask out the effects of fibrosis on US images. This phenomenon disallows us to accurately detect fibrosis when associated with steatosis
Even if the steatosis is succesfuly detected, imagistic models have to be built for this pathology.These models will help us find features that are unaffected by the presence of steatosis and to find features that will discriminate fibrosis regardless of steatosis grade.
More patients have to be examined in order to cover the entire spectrum of pathologies that affect the liver. Without having enough "samples" we will not be able to take into consideration some aspects of the diffuse diseases. As a result the software system will not be able to detect the diffuse pathologies.
Recent publications
Cristian Vicas, Sergiu Nedevschi, Monica Lupsor, Radu Badea, Horia Stefanescu, "Fibrosis detection from ultrasound imaging. The influence of necro-inflammatory activity and steatosis over the detection rates", Proceedings on Workshop on Computers in Medical Diagnoses WCMD, September 6-7, 2007
Cristian Vicas, Sergiu Nedevschi, Monica Lupsor, Radu Badea, Mircea Grigorescu, "Steatohepatitis Detection from Ultrasound Images Using Attenuation and Backscattering Coefficients", Proceedings on Workshop on Computers in Medical Diagnoses WCMD, September 6-7, 2007
Monica Lupsor, Radu Badea, Sergiu Nedevschi, Cristian Vicas, Simona Tripon, Horia Stefanescu, Corina Radu, Mircea Grigorescu, "The assessment of liver fibrosis using the computerized analysis of ultrasonographic images. Is the virtual biopsy appearing as an option?", Acta Electrotehnica, Proceedings of the 1st Internationa Conference on Advancements of Medicine and Health Care Through Technology Meditech, 2007, Volume 48, Number 4, 2007 p245-251
Monica Lupsor, Sergiu Nedevschi, Cristian Vicas, Radu Badea, Mircea Grigorescu, Simona Tripon, Horia Stefanescu, Corina Radu, Alexandru Serban,Titus Suteu, "Estimating the fibrosis stage in the human liver tissue using image processing methods on ultrasonographic images. Preliminary results.", Proceedings of the Euro-Mediterranean Medical Informatics and Telemedicine. 3rd International Conference, 2007: 311-318
Lupsor M, Badea R, Vicas C, Nedevschi S, Tripon S, Stefanescu H, Suteu T, Radu C, Grigorescu M, "Se pot diferentia stadiile fibrozei in hepatita cronica virala C utilizand examinarea ultrasonografica optimizata prin analiza computerizata a imaginii? Rezultate preliminare". Revista romana de Ultrasonografie, 2007; 9(1): 170-171
Lupsor M, Badea R, Vicas C, Nedevschi S, Tripon S, Stefanescu H, Suteu T, Radu C, Grigorescu M., "Estimarea stadiului fibrozei in hepatita cronica virala C folosind metode computerizate de analiza a imaginii ecografice. Rezultate preliminare", J Gastrointestin Liver Dis, 2007;16(suppl 1): 62-63.
References
[Yamada_2006] W. Yamada, M. Ebara, "A pilot approach for quantitative assessment of liver fibrosis using ultrasound: preliminary results in 79 cases", Journal of Hepatology vol. 44, pp. 68-75, 2006.
[Noble_2006] J.A. Noble , D. Boukerroui, "Ultrasound Image Segmentation: A Survey." IEEE Transactions on Medical Imaging, Vol 25, No 8, 987-1010, August 2006.
[Saadeh_2000] S. Saadeh, G. Cammell, W.D. Carey, Z. Younossi, D. Barnes, K. Easley, "The role of liver biopsy in chronic hepatitis C.", Hepatology, vol. 33, no 1, pp. 196-200, 2000.
[Poynard_2000] T. Poynard, V. Ratziu, P. Bedossa, "Appropriateness of liver biopsy." Can J Gastroenterol., vol. 14, no. 6, pp. 543-548, 2000.
[Yeh_2003] W.C. Yeh, H. Sheng-Wen, " Liver fibrosis grade classification with b-mode ultrasound", Ultrasound in Med. [[[&]]] Biol. , vol. 29, no. 9, pp. 1229-1235, 2003
[Ahmadian_2005] A. Ahmadian, A. Mostafa, "A texture classification method for diffused liver diseases using Gabor wavelets", Engineering in Medicine and Biology 27th Annual Conference Shanghai, China, September 1-4, 2005
[Lu_1999] Z.F. Lu, J.A. Zagzebski, F.T. Lee "U ltrasound Backscatter And Attenuation In Human Liver With Diffuse Disease" Ultrasound In Med. [[[&]]] Biol ., Vol. 25, No. 7, Pp. 1047-1054, 1999.